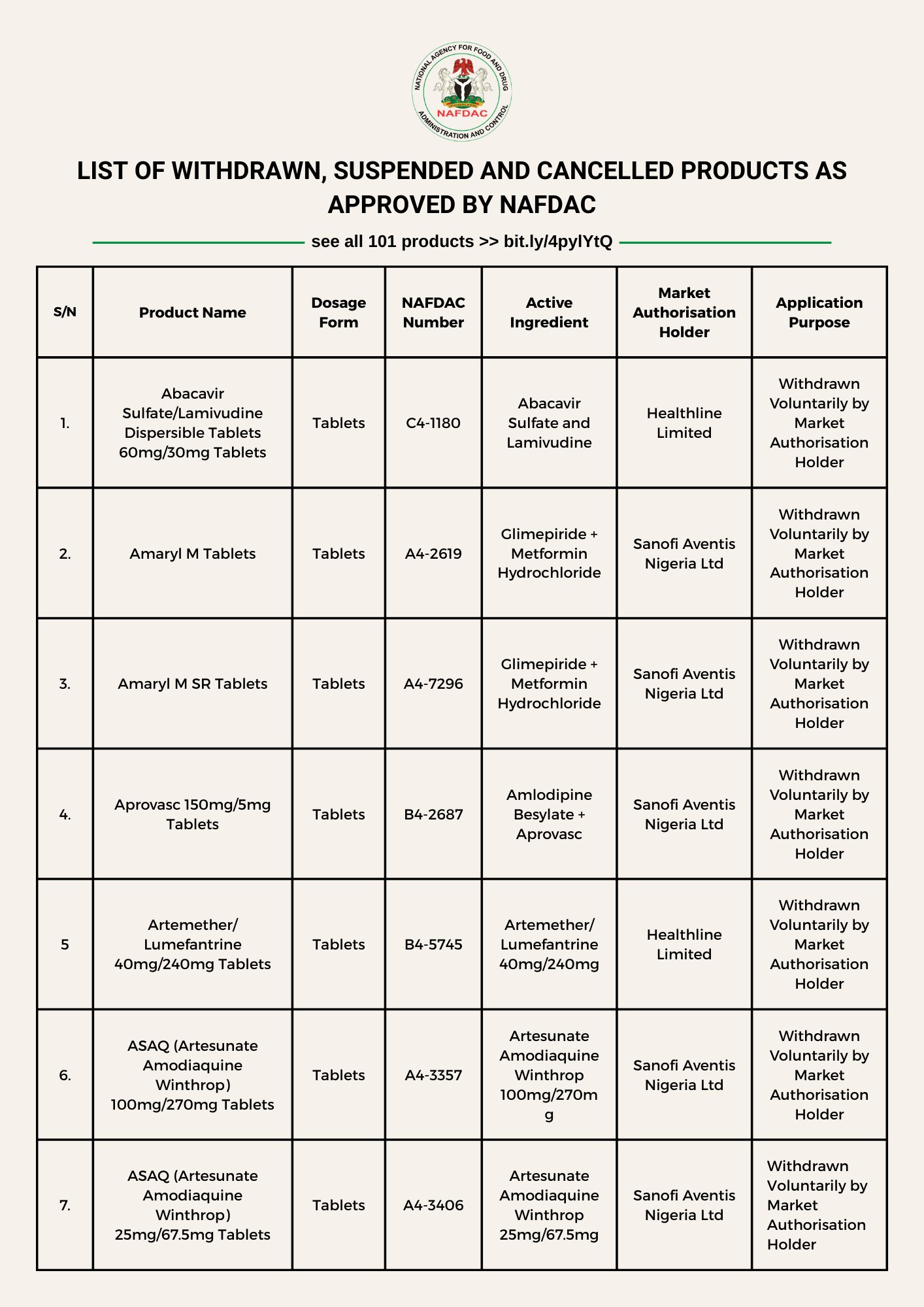
The National Agency for Food and Drug Administration and Control (NAFDAC) has mandated the immediate removal, suspension and cancellation of 101 products from the market.
NAFDAC disclosed this in a statement on Tuesday.
NAFDAC noted that the listed products “ are therefore no longer permitted for manufacture, importation, exportation, distribution, advertisement, sale and use within Nigeria. ”
It outlines three distinct regulatory actions.
NAFDAC stated “The Certificate of Registration of a product is said to be withdrawn when the use of the Certificate of Registration of that product is discontinued upon request of the Market Authorisation Holder.
“The Certificate of Registration of a product may be suspended when the conditions upon which the NAFDAC Registration license was issued are no longer met, and the Agency is to make a determination.
“The Certificate of Registration of a product is said to be cancelled when the NAFDAC Certificate of Registration license of that product is revoked by NAFDAC.”
See complete list (101 products): bit.ly/4pylYtQ